Which Statement Correctly Describes an Endothermic Chemical Reaction
The mixture instantly turns cool to the touch. B Two substances are dissolved in water and start to react.

What Are Endothermic Reactions With Examples Video
Which statement correctly describes an endothermic chemical reaction.

. Which type of reaction is the haber process. A A and C C BandC B A D D BandD. C It is exothermic and energy is absorbed.
A The products have higher potential energy than the reactants and H is negative. After 10 minutes the chemical reaction has reached equilibrium. Chemistry questions and answers.
She then adds chemical d and the reaction becomes hot and explodes. Which statement correctly describes an endothermic chemical reaction according to a plot of Energy and Reaction progress. The products are more stable than the reactants.
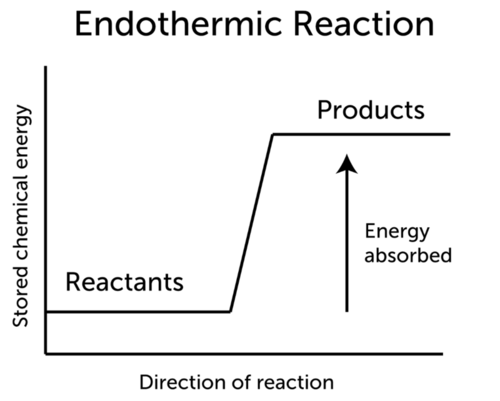
Select all that apply. The products have higher potential energy than the reactants and the ΔH is positive. The products have lower potential energy than the reactants and the H is negative.
What can be known about this situation. The products of an exothermic reaction are higher in energy than the reactants. Which option correctly describes the reactants and products of a chemical reaction.
In an endothermic reactionThe energy of the reactants is higher than the energy of the products. An exothermic reaction is one that releases heat. Cherylanne mixes together chemical a and chemical b in a test tube forming chemical c.
D is exothermic and energy is released. The products have lower potential energy than the reactants and the AH is positive The products have higher potential energy than the reactants and the AH is negative. Check all that apply.
Up to 24 cash back Which statement correctly describes an endothermic chemical reaction. Thus in a chemical equation or reaction products will have more energy than the. Which statement correctly describes an endothermic chemical reaction.
1 The products have higher potential energy than the reactants and the AH is negative. The products have lower potential energy than the reactants and the H is negative. E products have higher potential energy than the reactants and the AH is positive.
The products have higher potential energy than the reactants and the AH is negative. The products have higher potential energy than the reactants and the H is positive. ANYBODY HAVE Chemical Reaction Systems Online Practice Two substances are combined and react.
The products have lower potential energy than the reactants and the AH is negative. An absorption of heat and a decrease in entropy. 3 The products have lower potential energy than the reactants and the AH is negative.
The products have lower potential energy than the reactants and the AH is negative. Which statement correctly describes an endothermic chemical reaction. B It is endothermic and energy is released.
The products have higher potential energy than the reactants and the ΔH is negative. Which statement correctly describes an endothermic chemical reaction. The products have higher potential energy than the reactants and the H is negative.
What is the standard enthalpy of formation changeHf for HClg. Which statement correctly describes an endothermic chemical reaction. Does anyone have the answers for Calculating mass quick check.
Matter cannot be created or destroyed in a chemical reaction B. The products have lower potential energy than the reactants and the ΔH is negative. Above 0 degrees Celsius ice changes spontaneously to water according to the following equation.
The products have higher potential energy than the reactants and the AH is positive. If the container of a reaction becomes colder during the reaction the. The products have higher potential energy than the reactants and the H is negative.
The products have lower potential energy than the reactants and the AH is. In an endothermic process heat is transferred from the surroundings to the system. Which statement accurately describes the appearance of the solution when it.
Which of the following statements correctly describe exothermic and endothermic reactions. H-H 436 kJmol I-I 151 kJmol H-I. The products have higher potential energy.
Which statement correctly describes an endothermic chemical reaction. A It is endothermic and energ is absorbed. In endothermic reactions energy is absorbed by a system from the surroundings.
The products have lower potential energy than the reactants and the AH is negative. Reactants in a chemical reaction rearrange to form a new substance or substances C. None of the above.
1 The products have higher potential energy than the reactants and the AH is negative. Endothermic describes a chemical reaction that is accompanied by the absorption of heat or an organism that generates heat to maintain its temperature. The products have lower potential energy than the reactants and the AH is positive.
The products have higher potential energy than the reactants and the AH is negative. Calculate the changeH for the following reaction using the bond energies given below. Which statement correctly describes this reaction.
The products have higher potential energy than the. Which statement correctly describes an endothermic chemical reaction. The products have higher potential energy than the reactants and the AH is positive.
The products have higher potential energy than the reactants and the H is positive. In an endothermic chemical reaction reactants absorbs heat energy the products will have higher potential energy than reactants H products. The enthalpy change for the following reaction is -1846 kJ.
H2Os heat -- H2Ol The changes in H2Os involve. The energy of the reactants is lower than the energy of the products. NaCls -- Naaq Cl-aq the entropy of the system.
B The products have higher potential energy.

What Are Endothermic Reactions With Examples Video

Solved Which Statement Correctly Describes An Endothermic Chegg Com

Comments
Post a Comment